How AI agents are transforming MedTech compliance processes
Reading Time: 4 minutes

Around 50 % of MedTech companies say they lack full confidence in the completeness of their regulatory data for global product registrations.1
Regulatory complexity is inherent in every device, software update, and market launch in the MedTech industry. Whether navigating Medical Device Regulation (MDR) or the privacy/data-security obligations under the Health Insurance Portability and Accountability Act (HIPAA), MedTech companies must juggle multiple strings at once.
Enter AI Agents. Smart, autonomous systems, trained on regulatory frameworks, guidance documents, internal product data, and cross-functional workflows. They can act as transformative assistants in the compliance journey. They help surface non-compliance risks, automate document generation and tracking, and integrate workflows across functions.
Key data challenges in MedTech compliance
For MedTech companies, compliance is all about managing and governing massive volumes of data that flow through every stage of a product’s lifecycle. As devices become smarter and more connected, data complexity has become one of the biggest barriers to regulatory readiness.
- Managing fragmented and evolving regulatory data across geographies
Regulatory requirements differ widely across markets, from the FDA’s UDI (Unique Device Identification) to the EU’s MDR and IVDR reporting mandates. Each demands consistent, structured data that aligns with local formats. However, data often resides in silos across R&D, manufacturing, quality, and regulatory systems. - Ensuring data accuracy, traceability, and audit readiness
Data integrity lies at the core of every compliance submission. Yet, manual entries, disconnected databases, and inconsistent metadata often lead to version mismatches or incomplete audit trails. This lack of unified visibility can delay approvals or trigger costly observations during audits. - Monitoring compliance across the product data lifecycle
From design inputs and supplier data to clinical evaluations and post-market surveillance, every stage in the product lifecycle generates critical compliance data. Without centralized analytics, identifying anomalies, tracking adverse events, or linking field data to design records becomes a labor-intensive task. - Dealing with unstructured data
A significant portion of MedTech compliance data such as clinical notes, incident reports, or supplier communications exists in unstructured formats. Without automation or standardized frameworks, teams spend more time cleaning and reconciling data than analyzing it. This leads to delayed or inaccurate insights, compliance reporting errors, and potential financial penalties due to missed deadlines.
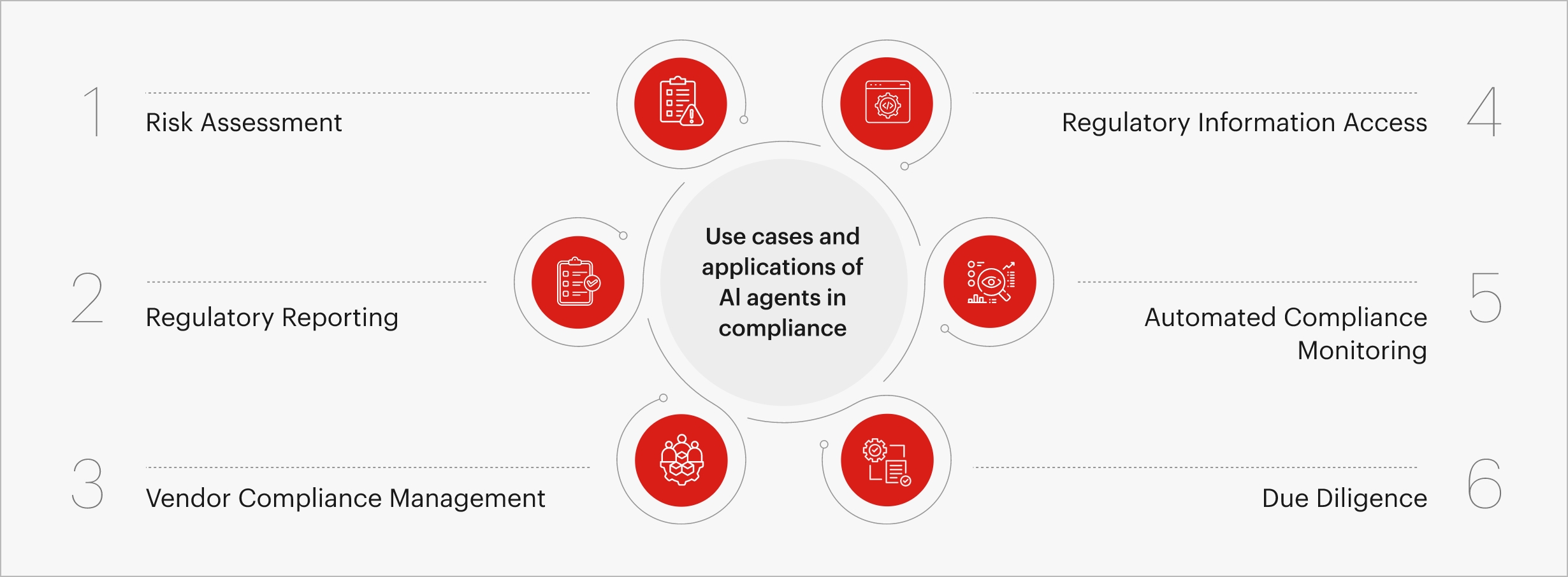
Fig 1. Applications of AI agents in compliance
What role do AI Agents play in MedTech compliance
As compliance demands become increasingly data-intensive, traditional manual processes can no longer match the scale and speed required. This is where AI agents, intelligent autonomous systems trained on regulatory frameworks, enterprise data, and domain-specific rules, are transforming the compliance landscape. By embedding intelligence directly into workflows, they help MedTech companies move from reactive to predictive compliance management.
- Automating routine compliance checks and documentation
AI agents can automatically review technical files, validation documents, and submission packages for completeness and accuracy. They cross-reference product data with regulatory templates and flag inconsistencies before they reach auditors. This automation significantly reduces manual review time, improves data integrity, and ensures every document meets the latest standards. - Real-time monitoring of regulatory updates and changes
With regulations evolving continuously across markets, staying updated is a major challenge. AI agents can monitor global regulatory databases, government portals, and industry bulletins in real time, detecting new rules, classification updates, or guidance revisions as they happen. - Intelligent risk assessment and reporting
AI-powered agents can analyze structured and unstructured data from manufacturing logs to complaint records. A vast amount of knowledge is often created but never analyzed in these unstructured formats. Using machine learning, they can connect field data with design or supplier inputs to identify emerging risks or potential compliance gaps. - Assisting in audit preparation and tracking corrective actions
Audits can be stressful and time-consuming. AI agents simplify this process by automatically generating audit-ready reports, tracking evidence trails, and maintaining an organized repository of documents mapped to specific regulations. After audits, they can assign and monitor corrective actions, ensuring closure and compliance verification within defined timelines.
Case Study: Regulatory compliance excellence in MedTech
Sigmoid helped one of the largest medical technology companies in the world optimize medical device reporting compliance using AI agents that reduced manual effort in the complaint handling process.
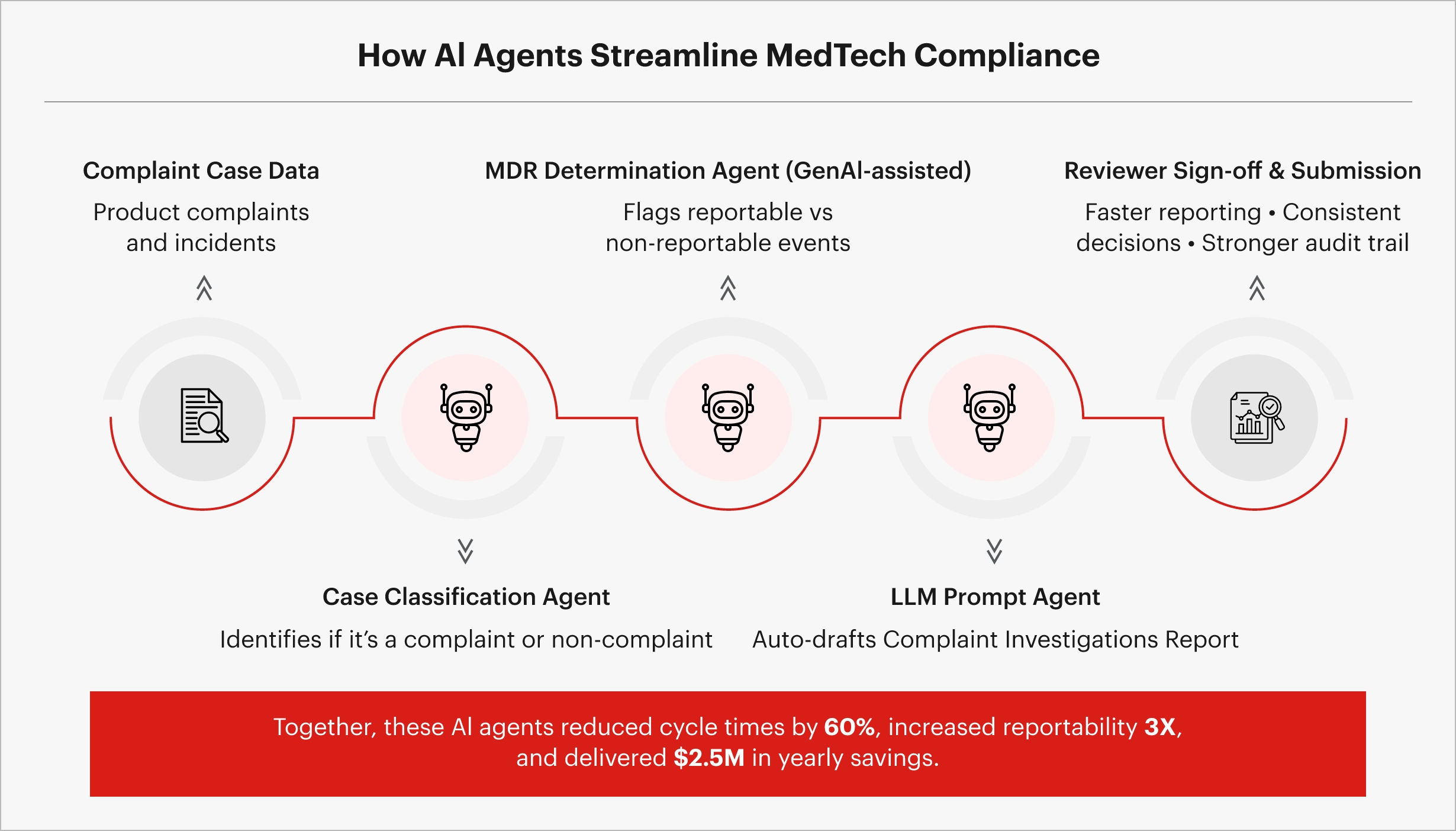
Fig 2. AI agents in action
Conclusion
MedTech compliance is shifting from reactive control to predictive intelligence. AI agents are making this possible by analyzing data patterns, anticipating regulatory changes, and automating compliance workflows. The next leap will be fully autonomous agents that manage compliance continuously and intelligently across global markets. But the real power lies in collaboration. Human experts bring judgment and ethical oversight, while AI brings speed and precision. Together, they redefine compliance as a strategic advantage
Featured blogs
Subscribe to get latest insights
Talk to our experts
Get the best ROI with Sigmoid’s services in data engineering and AI
Featured blogs
Talk to our experts
Get the best ROI with Sigmoid’s services in data engineering and AI